Abstract
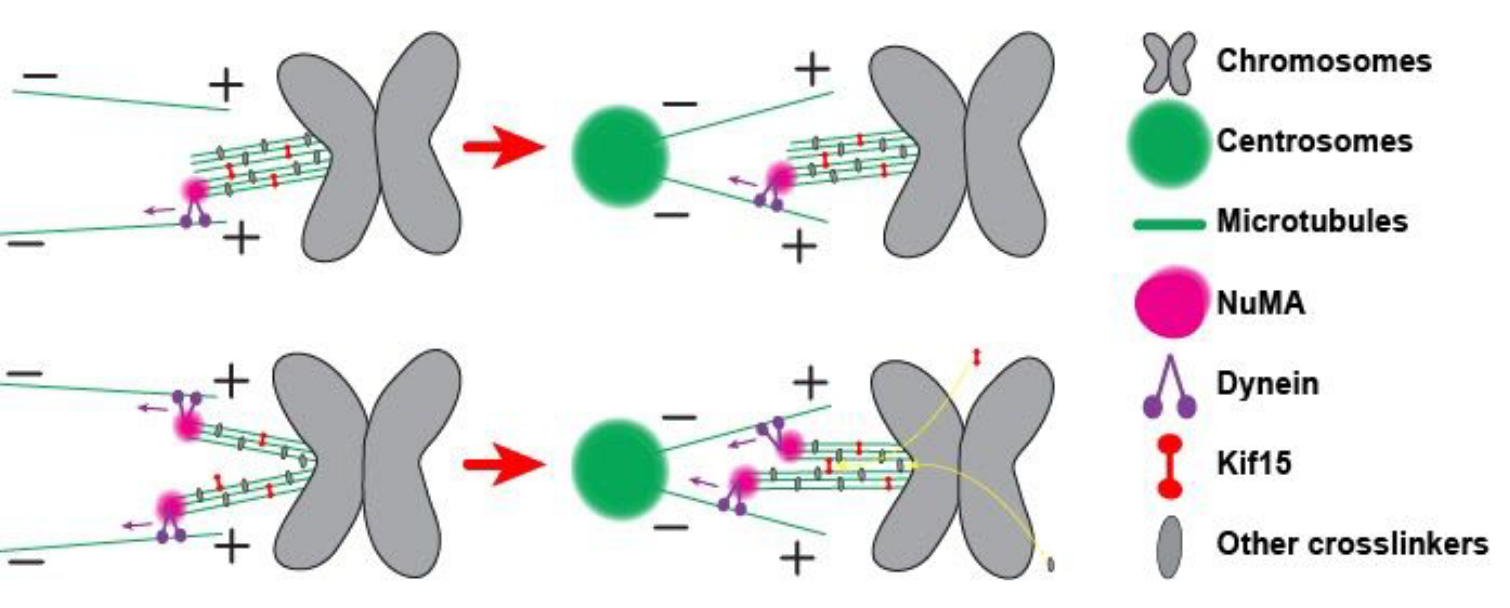
The mitotic spindle, a self-constructed microtubule-based machine, segregates chromosomes into two eventual daughter nuclei. In mammalian cells, microtubule bundles called kinetochore-fibers (k-fibers) anchor chromosomes to the spindle poles, vitally supporting spindle function. Yet, how they achieve mechanical integrity is not well-understood. We sever k-fibers using laser ablation, testing the contribution that forces at minus-ends make to k-fiber cohesion. We measure the physical response of the remaining kinetochore-bound segments. We observe that microtubules within ablated k-fibers often, but not always, splay apart from their minus-ends. Furthermore, we find that minus-end clustering forces induced in response to ablation seem at least partially responsible for k-fiber splaying. We also investigate the role of the putative k-fiber binding molecule kinesin-12 Kif15. We find that pharmacological inhibition of Kif15 microtubule binding reduces k-fiber mechanical integrity. In contrast, inhibition of its motor activity but not its microtubule binding does not greatly affect splaying. Altogether, the data suggest that forces holding k-fibers together are of similar magnitude to other spindle forces, and that Kif15, acting as a microtubule crosslinker, helps fortify and repair k-fibers.