Abstract
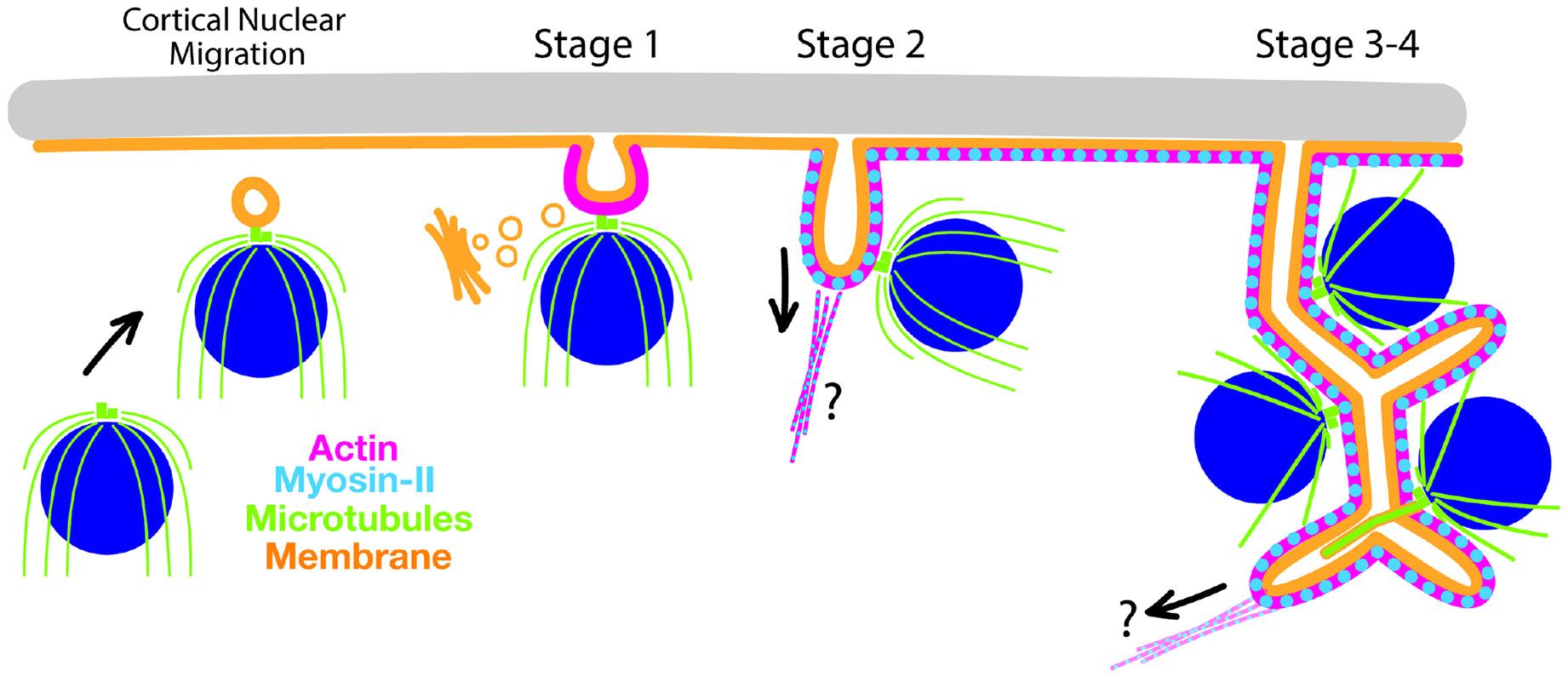
Chytrid fungi provide a model for studying foam-like cellularization, where nuclei that are dispersed throughout the cytoplasm are synchronously compartmentalized into daughter cells. This organization poses geometric challenges not faced by cells undergoing conventional cytokinesis or Drosophila monolayer cellularization, where nuclei are organized in linear or planar arrangements with ready access to the plasma membrane. We use the chytrid Spizellomyces punctatus to show that chytrid cellularization begins with migration of nuclei and their attached centrosomes to the plasma membrane, where centrosome-associated vesicles mark sites of membrane invagination. These vesicles then extend inward, resulting in tubular furrows that branch and merge to create a honeycomb of polyhedral membrane compartments-a cellularization foam-each with a nucleus and cilium. Using inhibitors and laser ablation, we show that tensile forces produced by actomyosin networks drive aphrogenesis (foam generation), while microtubules are important for foam patterning and ciliogenesis but are not essential for cellularization. Finally, we suggest that chytrids may have incorporated ancestral mechanisms associated with ciliogenesis to coordinate the association of internal nuclei with membrane furrows to solve the unique geometric challenges associated with aphrogenic cellularization.